Contact Us
South San Francisco CA Headquarters:
Quanta Therapeutics Inc.
2 Tower Place, 17th Floor
South San Francisco, CA 94080
Radnor PA Site:
Quanta Therapeutics Inc.
250 King of Prussia Road
Radnor, PA 19087
Developing best-in-class allosteric inhibitors for RAS-driven cancers
KRAS mutations and amplifications are a driver in nearly one-quarter of all cancers. We are developing allosteric small molecules to target previously undruggable RAS mutations, such as KRASG12D and KRASG12V, in difficult to treat cancers, including pancreatic, colorectal, lung and endometrial cancer types. KRAS inhibitors have been developed to target the KRASG12C mutation, but these therapies are limited to only around ten percent of KRAS-driven cancers.
We are focused on modulation of RAS signaling at the cell membrane to broaden the scope of treatable RAS-driven cancers. We are applying deep biologic insight, unique protein conformation detection technology, sophisticated medicinal chemistry, and clinical development expertise to advance multiple, chemically distinct KRAS inhibitor programs with differentiated mechanisms of action. Our technology and capabilities are enabling the development of best-in-class, oral, small molecules with the potential to lead the next wave of innovation in KRAS therapy.
We are an experienced team of RAS biology leaders, innovative medicinal chemists, and clinical development experts. The Quanta team brings together a scientific foundation based on discovery research from RAS signaling pioneers with extensive oncology-focused small molecule translational and clinical development experience. Collectively, our leadership team has contributed to the discovery, development, approval, and launch of multiple biopharmaceutical medicines.
Perry Nisen, MD PhD
Chief Executive Officer
Cameron Pitt, PhD
Chief Operating Officer
Leonardo Faoro, MD, MBA
Chief Medical Officer
Vanessa L. Jacoby, CPA
Chief Business and Financial Officer
Hong Lin, PhD
SVP of Drug Discovery
Juan Luengo, PhD
SVP of Chemistry
Derek Metzger
Chief Strategy Officer
Tina Woo
VP, Head of Development Operations
Mike Kramer
VP, Head of Scientific Operations
Elizabeth Donohue Vo, PhD
VP, Discovery Biology
David H. Rominger
Sr. Dir. of Nonclinical Drug Development
Chief Executive Officer
Perry became CEO of Quanta Therapeutics in June 2021 after having served as the founding Executive Chairman of the company during formation in his role as Executive Partner at Sofinnova Investments beginning in 2018. He is a physician scientist with extensive experience in drug discovery and development with particular interest in oncology. Perry is the former CEO of the Sanford Burnham Prebys Medical Discovery Institute (SBP). As CEO, he was responsible for all aspects of the organization including strategy, operations and performance. Prior to SBP, he held multiple senior leadership positions during his tenure at GlaxoSmithKline (GSK) including SVP of Science and Innovation, interim Chief Medical Officer, and Oncology Therapy Area Head. Responsibilities at GSK included all aspects of drug discovery, clinical development, risk/benefit and operations. Before GSK, Perry was the Divisional Vice President, Cancer Research then Oncology Clinical Development at Abbott Laboratories. Prior to industry he was the Lowe Foundation Professor at the University of Texas Southwestern Medical Center. Perry holds a BS from Stanford University, and MD/PhD from the Albert Einstein College of Medicine. He is also an independent Director of TEVA Pharmaceuticals, and chair of their science and technology committee.
Chief Operating Officer
Cameron joined Quanta Therapeutics in April 2020 as Chief Operating Officer. He leads operational strategy and business transactions. Previously as a Principal at Sofinnova Investments, he led the Series A investment in formation of Quanta. Prior to joining Sofinnova, he was an Investment Professional at Versant Ventures and focused on early stage therapeutics and platform company building, particularly in oncology, immunology and fibrosis. He managed operations of several biotech companies and academic programs associated with Versant’s discovery engines, Highline Therapeutics and Inception Sciences. During his PhD, Cameron worked Dr. Frank McCormick on new strategies targeting Ras in cancer, and also interned in Translational Oncology at Genentech. He received his PhD in Biomedical Sciences at UCSF as an NSF Fellow and inaugural UCSF Discovery Fellow. He earned his BS with Honors in Biology from Stanford University and was awarded the Firestone Medal, Stanford’s top honor for undergraduate research. Cameron is also a past fellow of the NIH Intramural Research Training Award program.
Chief Medical Officer
Leo joined Quanta Therapeutics as Chief Medical Officer in November 2022. Leo previously served as Senior Vice President and early clinical development lead at Exelixis, a commercial oncology-focused biotechnology company, where he was responsible for providing strategic leadership to expand the company’s early clinical pipeline and advancing its development candidates into the clinic. In his prior role as vice president of clinical development (late-stage) at Exelixis, Leo led the development of the company’s GI/Thyroid franchise for cabozantinib (Cabometyx®), including pivotal and post-marketing studies across oncology indications and regulatory approval filings, and achieving FDA approval of cabozantinib (Cabometyx®) in metastatic differentiated thyroid cancer under Breakthrough Designation. Prior to Exelixis, Leo served as Vice President of clinical development at OncoMed Pharmaceuticals, an oncology-focused biotechnology company, where he led the early clinical development of the company’s pipeline, including two first-in-human studies, through to its reverse merger with Mereo BioPharma. Leo began his clinical development career in positions of increasing responsibility at Genentech where he had global clinical development responsibility for bevacizumab (Avastin®), including in lung, prostate, breast and renal cell cancers. Leo earned a medical doctorate from the Universidade Federal do Parana in Brazil. He trained in internal medicine at the Mayo Clinic in Minnesota and completed a medical oncology fellowship at the University of Chicago. Additionally, Leo holds a Master's in Business Administration from Columbia University and the University of California, Berkeley.
Chief Business and Financial Officer
Vanessa joined Quanta in June of 2025. Prior to joining Quanta, Vanessa served as Chief Financial Officer of Shoreline Biosciences, where she played a key role in the marketing and closing of Shoreline’s $140M Series B round, as well as the establishment of partnerships with Kite Pharma and BeiGene, both of which included meaningful upfront payments and a combined total deal value of $3.7 billion. Prior to Shoreline, Vanessa served as Chief Accounting Officer of Avidity Biosciences, where she led corporate finance and planning activities and played a key role in the company’s initial public offering and cross-over financing. Previously, she held positions of increasing responsibilities at PharmAkea, which was acquired by Galecto in 2019, Brain Cells, Artes Medical and Verenium Corporation. Vanessa started her career as an auditor for Ernst & Young. She received her B.S. degree in Business Administration from Fundação Armando Alvares Penteado, Sao Paulo, Brazil and is a Certified Public Accountant with the State of California (inactive). She currently serves on the board of trustees for the Ruben H. Fleet Science Center in San Diego and is on the board for the Association of Bioscience Financial Officers (ABFO) Southwest chapter.
SVP of Drug Discovery
Hong is Senior Vice President of Drug Discovery at Quanta Therapeutics. Before joining Quanta, Hong was Executive Director, Head of Early Discovery at Prelude Therapeutics, where she led the PRMT5 program that delivered clinical candidates PRT543 and PRT811 within a two-year period. Prior to Prelude, Hong held multiple leadership roles in medicinal chemistry and virtual drug discovery groups within the therapeutic areas of oncology, neuroscience and regenerative medicine during her 15-year tenure at GlaxoSmithKline. Hong holds a Ph.D. degree in Organic Chemistry from the Brandeis University and did her postdoctoral training under an NIH fellowship at Memorial Sloan-Kettering Cancer Center.
SVP of Chemistry
Juan is Senior Vice President of Chemistry at Quanta Therapeutics. Juan leads chemistry strategy and structural modeling for drug discovery. Before joining Quanta, Juan was Founding Scientist and VP of Drug Discovery at Prelude Therapeutics, an oncology start-up company in Delaware. Prior to Prelude, Juan worked for 27 years at GSK, where he was Head of Chemistry in the Cancer Epigenetics group. During his 30+ years in pharmaceutical research, Juan has managed the progression of several compounds into clinical studies in different therapeutic areas. Most notably Juan was one of the inventors of Promacta (eltrombopag), and he successfully led this first-in-class TPO Receptor agonist agent to the clinic. Juan holds a Ph.D. degree in Organic Chemistry from the University of Michigan and did postdoctoral research at Stanford University.
Chief Strategy Officer
Derek Metzger is the Chief Strategy Officer at Quanta. Derek has worked in oncology drug development for 15 years, most recently as the Executive Director of Strategy at Tempest Therapeutics. Prior to Quanta and Tempest, Derek worked at Aduro Biotech, and Onyx Pharmaceuticals and has held roles spanning many different functions, including finance, business development, strategic planning, program leadership, and commercial. Derek graduated from Brown University with a degree in Economics and has his M.S. degree in Biotechnology from Johns Hopkins University.
VP, Head of Development Operations
Tina is the Vice President and Head of Development Operations at Quanta. Tina was most recently with Calithera Biosciences, an oncology-focused biopharmaceutical company, where she was Head of Clinical Operations and instrumental in building the company’s infrastructure to allow the initiation and execution of Phase 1 to Phase 3 clinical studies. While with Calithera, Tina was also involved in establishing and providing oversight to multiple functions, including Pharmacovigilance and Data Management. Tina brings over 20 years of industry experience across roles with both large and small biotechnology companies and with increasing levels of responsibility. Prior to Calithera, she was at Onyx Pharmaceuticals, Cell Biosciences, Celera Diagnostics, Exelixis, and Rigel. Tina holds a B.S. in Biology from San Francisco State University.
VP, Head of Scientific Operations
Mike Kramer, Vice President, Head of Scientific Operations at Quanta Therapeutics, has over 30 years of experience in pharmaceutical and biotechnology research and development, overseeing nonclinical development, CMC, regulatory, and project management activities. Prior to joining Quanta, Mike spent 10 years at Trevena Inc., most recently as Vice President of Scientific Operations, Regulatory and Alliance Management, developing novel drugs targeting G protein-coupled receptors for the treatment of cardiovascular and CNS diseases. Prior to joining Trevena, Mike served as Director of Nonclinical Pharmacology and Toxicology at Synergy Pharmaceuticals, where he worked on the development of novel small molecules/peptides for the treatment of gastrointestinal diseases. Prior to that role, Mike held roles of increasing responsibility within Nonclinical Development and Safety at Neuron Therapeutics, Palatin Technologies and Promedior, developing drugs in multiple therapeutic areas ranging from fibrotic disease to CNS conditions. Mike received his M.S. degree in Molecular Biology from Drexel University.
VP, Discovery Biology
Elizabeth leads the biology team at Quanta Therapeutics. Prior to Quanta, Elizabeth worked at Biodesy where she developed conformation-based assays (SHG) using second-harmonic generation on a variety of protein targets. Elizabeth has deep experience in small molecule screening and cancer biology. She completed postdoctoral training at UCSF with Frank McCormick, where she designed and carried out a number of RAS-targeted small molecule campaigns using emergent technologies including SHG. Elizabeth has a Ph.D. in biochemistry and molecular biology from the University of British Columbia and a Bachelor of Science Honors degree in biochemistry from McGill University.
Sr. Dir. of Nonclinical Development
Dave Rominger, Senior Director of Nonclinical Drug Development, has over 35 years of experience in drug discovery and development at both large pharmaceutical and small biotech companies with a focus on leading IND-enabling activities. Prior to joining Quanta, he was Director of Drug Metabolism and Analytical Sciences at Prelude Therapeutics, where he member of the discovery and non-clinical development teams responsible for leading strategy and conduct of DMPK, safety pharmacology and general toxicology studies. Prior to Prelude, Dave was Associate Director of Non-clinical Development at Trevena, where he was responsible for support of G protein-biased ligand drug discovery screening, assay development and leading external non-clinical development activities, including DMPK and safety pharmacology, and he was an Investigator in the Enzymology and Mechanistic Pharmacology Department, Oncology Center of Excellence in Drug Discovery at GlaxoSmithKline. Before joining GSK, Dave was a Senior Research Scientist in the CNS Diseases department for 17 years at DuPont, where he supported drug discovery programs related to several therapeutic areas in neuroscience. Dave received his B.S. in Biology from The Catholic University of America.
Perry Nisen, MD PhD
CEO, Director
Cameron Pitt, PhD
COO, Director
Maha Katabi, PhD
Director
Rajul Jain, MD
Director
Maria Fardis, PhD MBA
Director
Ying Huang, PhD
Director
Frank McCormick, PhD FRS
UCSF
Andrej Sali, PhD
UCSF
Ben Cravatt, PhD
Scripps
Phil Baran, PhD
Scripps








Despite the approval of KRASG12C inhibitors, there remains a large patient population that may benefit from inhibition of other RAS mutations, particularly KRASG12D and KRASG12V, which are highly prevalent in cancers of the colon, pancreas and lung.
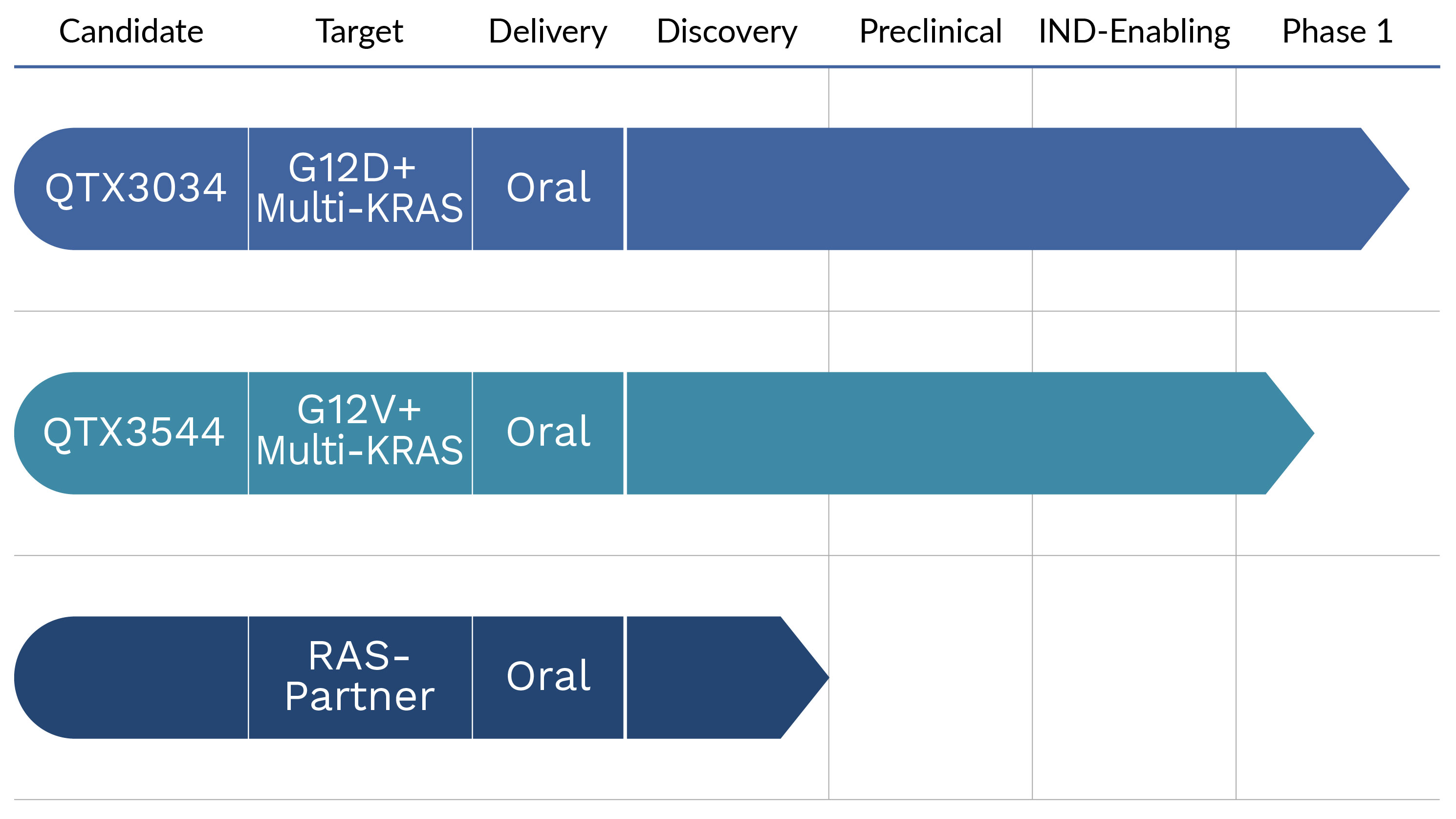
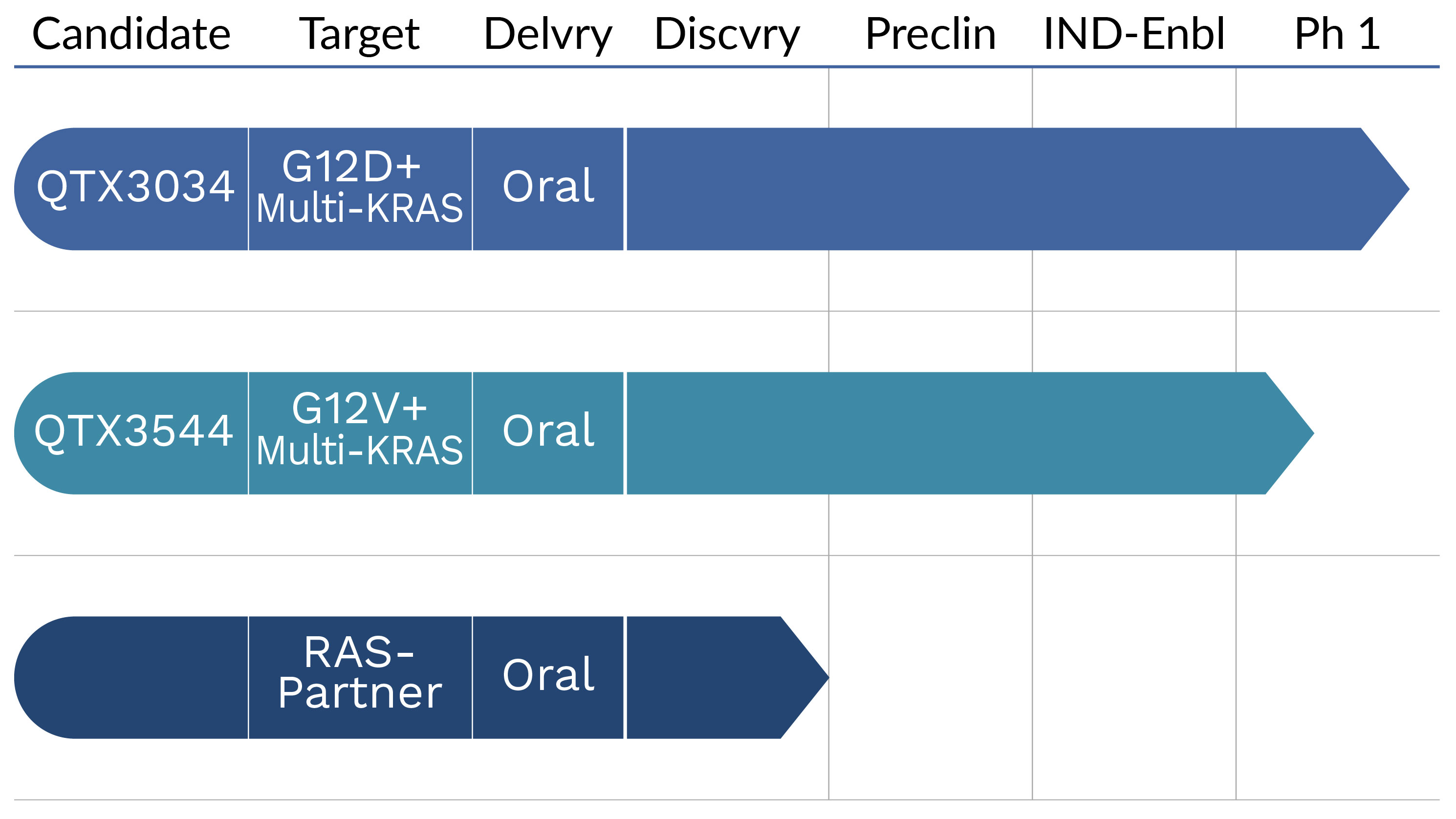
Our pipeline includes KRAS inhibitor programs from distinct chemical series, including a G12D-preferring multi-KRAS candidate (QTX3034) and a G12V-preferring multi-KRAS (QTX3544) currently being evaluated in Phase 1 clinical trials. Based on favorable safety and encouraging preliminary efficacy, QTX3034 has progressed into dose expansion cohorts in KRASG12D-mutant pancreatic, colorectal, and endometrial cancers. In preclinical studies, our candidates have demonstrated potent, selective, oral, CNS-penetrant properties. These data support the potential for our pipeline to significantly expand the scope of treatable KRAS-mutated cancers with direct, oral therapies.
Our unique conformation-sensitive Second Harmonic Generation (SHG) assays enable direct allosteric modulation of signaling complexes in the mitogen-activated protein kinase (MAPK) pathway to target a broad spectrum of RAS-driven tumors. These programs have potential as both monotherapies and synthetic lethal combination partners enhancing Quanta’s pipeline.


October 24, 2025
Quanta Therapeutics Presents Positive Phase 1 Data for QTX3034, an Oral G12D-Preferring Multi-KRAS Inhibitor, in Patients with Advanced Solid Tumors
july 23, 2025
Quanta Announces Leadership Team Appointment and Highlights Clinical Program Progress
April 28, 2025
Quanta Therapeutics Presents Late-Breaking Data for QTX3544, an Oral G12V-Preferring Multi-KRAS Inhibitor, at AACR Annual Meeting 2025
January 8, 2025
Quanta Announces IND Clearance by U.S. FDA for QTX3544, an Oral G12V-Preferring Multi-KRAS Inhibitor, and Other Pipeline Updates
January 4, 2024
Quanta Announces IND Clearance by U.S. FDA for QTX3034, G12D-Preferring Multi-KRAS Inhibitor, and Other Pipeline Updates
October 11, 2023
Quanta Therapeutics Presents Late-Breaking Data for QTX3034, a multi-KRAS inhibitor, at AACR-NCI-EORTC Conference
May 22, 2023
Quanta Therapeutics Raises Over $50 Million in Series D Financing to Advance its Pipeline of KRAS Inhibitors
April 14, 2023
Quanta Therapeutics Presents Data from KRAS Inhibitor Pipeline at Annual AACR Meeting in Late-Breaking Research Session
March 14, 2023
Quanta Therapeutics Announces Two Late-Breaking Preclinical Presentations from KRAS Inhibitor Pipeline at Annual AACR Meeting
December 13, 2022
Quanta Therapeutics Appoints Leonardo Faoro, MD, MBA, as Chief Medical Officer
October 26, 2021
Quanta Therapeutics Announces Oversubscribed $60 Million Series C Financing to Advance Novel RAS-targeting Programs
When we set out to make a superior class of medicines to address RAS-driven cancers, we intentionally created a work environment that was designed to foster that mission. A place where incredibly talented individuals are empowered to do their best work. As Quanta continues to grow, we will be guided by the principles that define us and we hope that they will serve each new employee who joins our team.
We are seeking highly motivated drug-hunters to join our passionate and experienced scientific team.
Please be alert to potential fraudulent recruiting efforts, such as when an individual not associated with Quanta Therapeutics pretends to be someone from Quanta. If you have been contacted about a role, please reach out to heather.meeks@quantatx.com to verify the authenticity of the position.
There are no positions available at this time.